Introduction
Desulfurization is essential for reducing the sulfur content in petroleum products. High sulfur levels not only lead to the formation of sulfur dioxide during combustion, which is a major contributor to air pollution and acid rain, but also cause corrosion in pipelines and engines. Hydrogenation, on the other hand, is used to add hydrogen atoms to unsaturated hydrocarbons, improving the quality and stability of fuels.
Ammonium molybdate, with its unique chemical properties, has found widespread application in these two key processes. Its ability to facilitate chemical reactions makes it an indispensable part of modern petrochemical production. In the following sections, we will delve deeper into how ammonium molybdate functions in desulfurization and hydrogenation processes, exploring its mechanisms, advantages, and challenges.

Ammonium Molybdate: An Overview
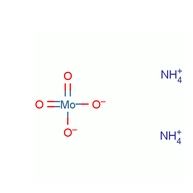
Ammonium molybdate, with the chemical formula H8MoN2O4, is a white crystalline powder that is soluble in water, acids, and alkalis but insoluble in alcohol. It has a density of 2.498 g/cm³ and decomposes at 170°C, releasing ammonia, water, and molybdenum trioxide.
The structure of ammonium molybdate is crucial to its catalytic properties. The molybdenum atom in the molybdate anion has a +6 oxidation state, which allows it to participate in redox reactions. The ability of molybdenum to change its oxidation state readily between +6, +5, and +4 enables ammonium molybdate to act as an effective catalyst. It can accept and donate electrons during chemical reactions, facilitating the transformation of reactants into products.
In addition, the ammonium cations in the structure can interact with other molecules in the reaction system, influencing the reaction environment and the adsorption of reactants onto the catalyst surface. This interaction can enhance the reactivity of the catalyst and improve the efficiency of the chemical processes. Overall, the unique chemical structure and properties of ammonium molybdate make it a suitable catalyst for the demanding processes in the petrochemical industry.
The Role in Desulfurization Processes

1. The Significance of Desulfurization in Petrochemicals
In the petrochemical industry, desulfurization is of utmost importance. Sulfur compounds are commonly present in crude oil and petroleum products. When these sulfur - containing substances are burned, sulfur dioxide (\(SO_2\)) is released into the atmosphere. \(SO_2\) is a major precursor of acid rain, which can cause damage to forests, lakes, and buildings. It also contributes to air pollution, leading to respiratory problems for humans.
Moreover, sulfur compounds can cause corrosion in pipelines, storage tanks, and engines. This corrosion not only reduces the lifespan of these facilities but also increases maintenance costs. For example, in refineries, pipelines carrying high - sulfur oil need to be inspected and repaired more frequently due to the corrosive nature of sulfur compounds. By reducing the sulfur content in petroleum products, desulfurization can improve the quality of fuels, making them more suitable for use in modern engines that require cleaner fuels for optimal performance.
2. How Ammonium Molybdate Facilitates Desulfurization
Ammonium molybdate acts as a catalyst in desulfurization processes by lowering the activation energy of the reactions involved. In the presence of ammonium molybdate, the sulfur - containing compounds in petroleum can be more easily converted into less harmful substances.
The molybdenum in ammonium molybdate can change its oxidation state during the reaction. It can accept electrons from the sulfur - containing reactants, facilitating their transformation. For instance, in the presence of hydrogen and ammonium molybdate, thiophenic sulfur compounds can be hydrogenated. The molybdenum in the catalyst first adsorbs the sulfur - containing molecule on its surface. Then, through a series of redox reactions, the sulfur - carbon bond is broken, and hydrogen atoms are added to the sulfur and carbon atoms. This process is accelerated by the ability of molybdenum to cycle between different oxidation states, such as +6, +5, and +4. The ammonium ions in ammonium molybdate can also play a role by providing a suitable reaction environment and interacting with the reactants or other species in the reaction system.
Function in Hydrogenation Processes

The Catalytic Action of Ammonium Molybdate in Hydrogenation
Ammonium molybdate plays a crucial role in hydrogenation reactions by facilitating the activation of hydrogen and the addition of hydrogen atoms to the unsaturated bonds of hydrocarbons. The molybdenum in ammonium molybdate can interact with hydrogen molecules. The high - oxidation - state molybdenum in the catalyst can accept electrons from the hydrogen molecule, breaking the \(H - H\) bond and forming reactive hydrogen species on the catalyst surface. These activated hydrogen species are more reactive than molecular hydrogen and can more readily react with the unsaturated hydrocarbons.
It also helps in changing the reaction pathway. In the absence of a catalyst, the reaction between hydrogen and unsaturated hydrocarbons may have a high activation energy, making the reaction slow or even non - feasible under normal conditions. Ammonium molybdate provides an alternative reaction pathway with a lower activation energy. The unsaturated hydrocarbon molecules can adsorb onto the surface of the ammonium molybdate catalyst, where they come into close proximity with the activated hydrogen species. This allows for an easier addition of hydrogen atoms to the unsaturated bonds, promoting the formation of saturated hydrocarbons. For example, in the hydrogenation of benzene (\(C_6H_6\)) to cyclohexane (\(C_6H_{12}\)), ammonium molybdate enables the reaction to occur at a lower temperature and pressure, which is more energy - efficient and cost - effective for industrial production.
2. Practical Applications and Benefits
In practical petrochemical production, the use of ammonium molybdate as a catalyst in hydrogenation has several applications and benefits. In the production of lubricating oils, hydrogenation with ammonium molybdate as a catalyst can improve the quality of the base oils. It can remove sulfur and nitrogen impurities, as well as saturate the aromatic compounds present in the base oils. This results in lubricating oils with better oxidation stability, lower volatility, and improved viscosity - temperature properties.
Another application is in the production of jet fuels. The hydrogenation process catalyzed by ammonium molybdate can enhance the stability and combustion performance of jet fuels. By reducing the content of unsaturated hydrocarbons and sulfur - containing compounds, the fuel becomes more resistant to oxidation and produces less harmful emissions during combustion.
The use of ammonium molybdate in hydrogenation processes also brings economic benefits. It can increase the yield of desired products. For example, in the hydrocracking of heavy - oil fractions, the presence of ammonium molybdate as a catalyst can increase the yield of lighter, more valuable hydrocarbons such as gasoline and diesel. This increases the overall profitability of the refinery. Additionally, the improved quality of the products can also lead to higher market prices, further enhancing the economic viability of the petrochemical operations.
Comparison and Advantages

In terms of activity, ammonium molybdate shows high catalytic activity in both desulfurization and hydrogenation processes. For example, compared to some traditional metal - based catalysts like nickel - based catalysts in desulfurization, ammonium molybdate can achieve a higher conversion rate of sulfur - containing compounds at relatively lower temperatures. This is because the unique structure of ammonium molybdate allows for more efficient adsorption and activation of reactant molecules. The molybdenum atom with its variable oxidation states can quickly participate in redox reactions, promoting the reaction progress.
Regarding selectivity, ammonium molybdate can selectively target specific reactions. In hydrogenation, it can preferentially hydrogenate certain unsaturated hydrocarbons while leaving others relatively unchanged. This is in contrast to some non - selective catalysts that may cause over - hydrogenation or side reactions. For instance, in the hydrogenation of a mixture of alkenes and alkynes, ammonium molybdate can be tuned to hydrogenate the alkynes first, achieving a high - selectivity transformation.
Stability is another strong point of ammonium molybdate. It can maintain its catalytic performance over a relatively long period under typical petrochemical process conditions. In high - temperature and high - pressure environments, which are common in the industry, ammonium molybdate does not easily decompose or lose its catalytic activity. This is in contrast to some organic - based catalysts that may degrade under such harsh conditions.
Cost - effectiveness is also an advantage of ammonium molybdate. Although the initial cost of ammonium molybdate may seem moderate, considering its high activity and long - term stability, it can lead to significant cost savings in the long run. Since it can reduce the reaction temperature and pressure requirements, it cuts down on energy consumption. Additionally, its long - lasting catalytic activity means less frequent catalyst replacement, further reducing operational costs. Overall, these advantages make ammonium molybdate a preferred choice in many petrochemical desulfurization and hydrogenation processes.
Conclusion
Ammonium molybdate plays a crucial role in the desulfurization and hydrogenation processes of the petrochemical industry. Its unique chemical structure and properties enable it to effectively catalyze these reactions, reducing sulfur content in fuels and enhancing the quality and stability of petrochemical products.
The advantages of ammonium molybdate, such as high activity, selectivity, stability, and cost - effectiveness, make it a preferred catalyst in many industrial applications. However, challenges like catalyst deactivation and complex recycling processes still need to be addressed.
Looking ahead, continued research and development in improving catalyst stability and developing more efficient recycling technologies are expected. As the petrochemical industry continues to evolve towards more sustainable and efficient production methods, ammonium molybdate is likely to maintain its significance, and further exploration of its potential applications may lead to even greater advancements in the field.













